In order to get to know the thermal phenomena, it is necessary to pay attention to the inward structure of the substance. The substance consists of molecules that move. In the case of gases and liquids, molecules move freely at high speed. This movement is called Braun’s movement. Due to such movement there are multitude collisions, in which the molecules change their velocity and some of their energy during the collision.
When the gas is heated by particles in the gas move more intensely because the atoms move faster and take up more space. Therefore, such motion of the molecule can be called a thermal movement. Thermal movement can not be stopped, and even at the lowest temperatures the molecules slowly oscillate around their equilibrium positions. As the body temperature gets higher the particles move faster.
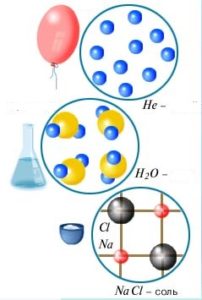 Thermal movement is present in all bodies. Many of these phenomena testify to this movement such as the movement of smoke through the room, the flow of air and the like. All particles within a body move and such heat movement is called the internal movement. The energy that the body has due to this movement is called the internal energy of the body. The internal energy is the sum of the total kinetic energy of the molecules and the potential interaction of all the molecules of the body.
Thermal movement is present in all bodies. Many of these phenomena testify to this movement such as the movement of smoke through the room, the flow of air and the like. All particles within a body move and such heat movement is called the internal movement. The energy that the body has due to this movement is called the internal energy of the body. The internal energy is the sum of the total kinetic energy of the molecules and the potential interaction of all the molecules of the body.
![]()
Hot water has higher energy than the same amount of cold water. By heating the body, the rate of movement of its particles increases, that is, the mean kinetic energy of the molecule increases. By heating, the temperature of the body increases. Thus, the temperature of the body is proportional to the mean kinetic energy of the molecule. The average kinetic energy and the temperature are two different quantities and have different measurement units.
In the lectures about mechanics we learned that mechanical work can change the energy of the body. Also, the internal energy of the body can be changed by mechanical work. Energy can be transferred between two bodies without performing any work by heat exchange. Heat is a part of the internal energy of a substance that passes from a warmer to a cooler body. When heat is released, internal energy decreases, and vice versa. Heat as a form of energy has two basic characteristics, one called temperature, and the second is the amount of heat.