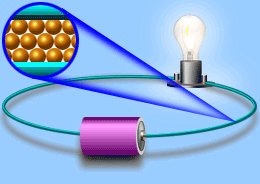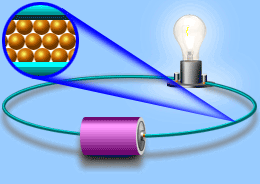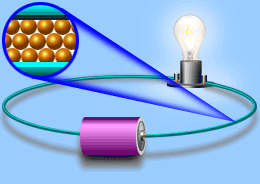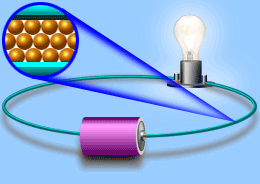
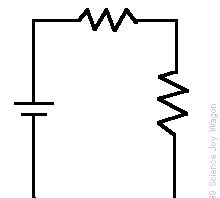
Electric current
Electricity is produced when an electron moves after being taken away from an atom. Review that atoms make up all matter. Atoms are usually electrically balanced, there are as many positive charges (protons) as there are negative charges (electrons). Particles with the same charge repel or push each other apart. Electrons repel electrons; protons repel protons. Particles with the opposite charges attract each other. However, when the electrons leave and move together it produces electricity. When this electricity is controlled, it is called current electricity, when it can’t be controlled it is called static electricity. We mentioned it article on capacitors.
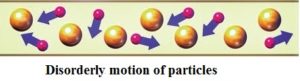
Electrons are always moving In static electricity, the electrons gather in one place and move randomly in all directions.

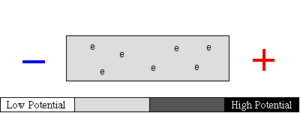
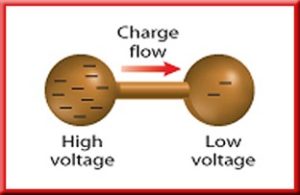
Example: With static electricity, charges flow between two balls during an electrostatic discharge. However, these charges follow an unpredictable path and it occurs for only a very short period of time.
 Example 2:
Example 2:
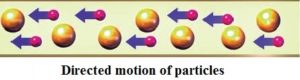
– A water stream is the movement of water particles in one direction and direction.
– The traffic flow is the movement of a car.
– Electric current is directed to the movement of charged particles.
Current In current electricity, there is a steady flow of electric charge (electrons) through a conductor. In current electricity, the charges move steadily for a much longer period of time This steady flow can be controlled and used to power various electrical devices. Recall that electrons move easily through conductors.
 Pressure – voltage
Pressure – voltage
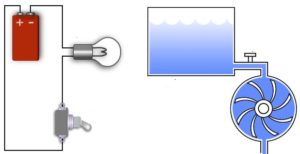
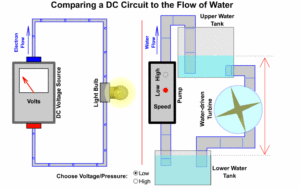
Figure shows a full water tank. This is where the water pressure is stored. The greater the amount of water in the tank, the greater the water pressure. The water tank in Figure can be compared to the battery in Figure, where a battery in an electrical circuit stores the electrical pressure (voltage). An empty tank of water with no pressure is similar to a flat battery with no electrical pressure.
Flow – current
Turning on the tap in Figure allows water, pushed out of the tank by pressure, to flow through the pipe and water wheel. This causes the wheel to rotate. Similarly, in Figure, turning on the switch allows current flow, pushed out of the battery due to electrical pressure, through the wire and globe.
“Current intensity” or “Current strength”
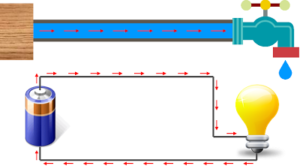
 The flow of an electric current in a wire is analogous to the flow of water in a pipe. The rate at which water flows through a pipe is given as the number of gallons (or cubic feet, or whatever) passing a particular point in the pipe per second. In the same way, the rate at which current flows in a wire is given as the number of coulombs of charge that passes through a cross-section of the wire per second. It is customary to say that the current (or “current intensity” or “current strength”) in a wire. The size of a stream is thus not conceptually equivalent to its flow rate. If q coulombs of charge pass through a cross-section of a wire in t seconds, then the current I in the wire is given by
The flow of an electric current in a wire is analogous to the flow of water in a pipe. The rate at which water flows through a pipe is given as the number of gallons (or cubic feet, or whatever) passing a particular point in the pipe per second. In the same way, the rate at which current flows in a wire is given as the number of coulombs of charge that passes through a cross-section of the wire per second. It is customary to say that the current (or “current intensity” or “current strength”) in a wire. The size of a stream is thus not conceptually equivalent to its flow rate. If q coulombs of charge pass through a cross-section of a wire in t seconds, then the current I in the wire is given by
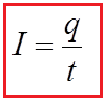
The rate of flow of current in a wire is given in amperes where one ampere represents a flow of one coulomb of charge per second. Thus if the flow rate of current at some point in a wire is three amperes, it means that three coulombs of charge is flowing past the point per second. Although an ampere represents a current flow of one coulomb per second.
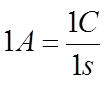

By the time the electron was discovered the idea of electricity flowing from positive to negative (conventional current) was firmly established. Luckily it is not a problem to think of electricity in this way because positive charge flowing forwards is equivalent to negative charge flowing backwards.
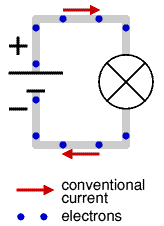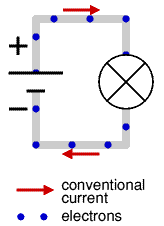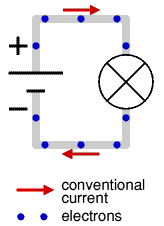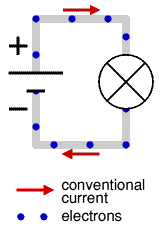
To prevent confusion you should always use conventional current when trying to understand how circuits work, imagine positively charged particles flowing from positive to negative.
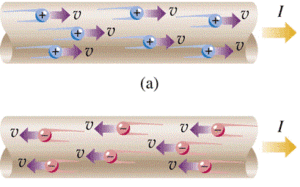
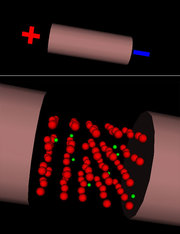
 Electric current can be generated in solid, liquid and gas environments, even in vacuum. Powered electric charges that can produce electric current are: electrons, positive and negative ions.
Electric current can be generated in solid, liquid and gas environments, even in vacuum. Powered electric charges that can produce electric current are: electrons, positive and negative ions.
– In conductors, freely convertible electrons are electrons (valence electrons, which belong to the external electron shell). An important category: metal conductors – valence electrons are very loosely bonded to the parent atoms and can easily leave them.
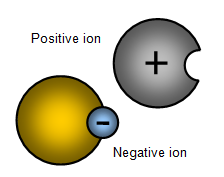
– Electrolytes are of particular importance in fluid environment
– Gases are, as a rule, good insulators
– Under certain conditions, there may also be an electrical current (neon tubes and fluorescent lamps).
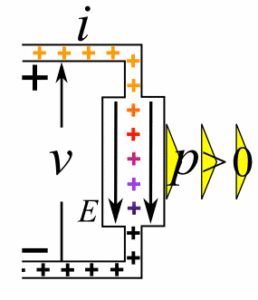
Def. Electromotive force (emf). The potential difference, or voltage, developed by any source of electrical energy such as a voltaic cell, storage battery, electrical generator, solar cell, thermocouple, piezoelectric cell, etc.The flow of water is similar to the flow of current. Water pressure stored in the tank is similar to voltage (electrical pressure) stored in a battery.
![]()


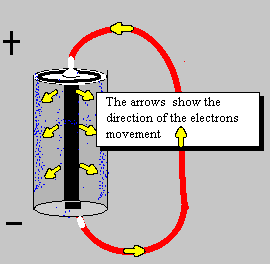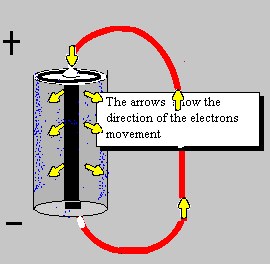
A source of electrical power can be a battery.
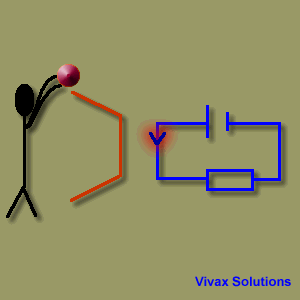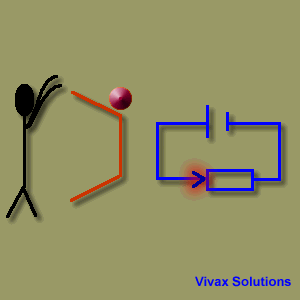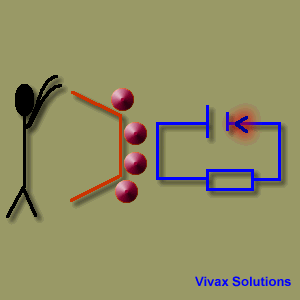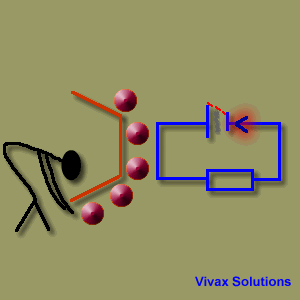 A source of emf can be thought of as a kind of charge pump that moves charge through its interior from a point of low potential to a point of high potential. The source of emf, by chemical, mechanical or other means, performs work on that charge to move it to the high potential terminal. The emf of the source is defined as the work done per unit charge in moving a small test charge from the point of low potential to the point of high potential i.e. if dW is the work done in moving test charge dq from the point of low potential to the point of high potential, then the emf E is given by
A source of emf can be thought of as a kind of charge pump that moves charge through its interior from a point of low potential to a point of high potential. The source of emf, by chemical, mechanical or other means, performs work on that charge to move it to the high potential terminal. The emf of the source is defined as the work done per unit charge in moving a small test charge from the point of low potential to the point of high potential i.e. if dW is the work done in moving test charge dq from the point of low potential to the point of high potential, then the emf E is given by
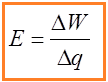
Emf is measured by the potential difference between the terminals when the battery or generator is not delivering current. In the mks system, the unit of emf is the volt (1 joule per coulomb).
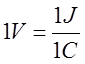
A source of emf is often called a seat of emf.
Are Voltage and Current Related?
Voltage and current are not the same thing, although they are closely related. In simple terms, Voltage causes Current. Given a Voltage and a path for the electrons, current will flow. Given the path, but no Voltage, or Voltage without the path, there will be no current.
A voltmeter is an instrument for measuring electrical potential in units of Volts.

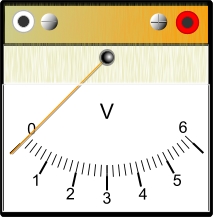
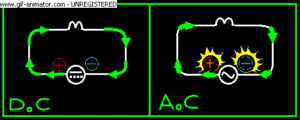
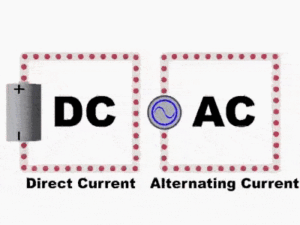
There are two types of electric current: direct current (DC) and alternating current (AC). The electrons in direct current flow in one direction. The current produced by a battery is direct current. The electrons in alternating current flow in one direction, then in the opposite direction—over and over again. In Canada and the United States, the current flow alternates 120 times per second. (In Europe it alternates 100 times per second.) The current supplied to your home by the local utility is alternating current.
Primary Batteries
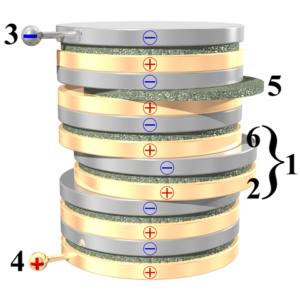


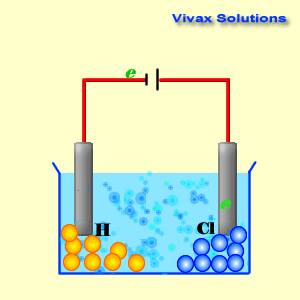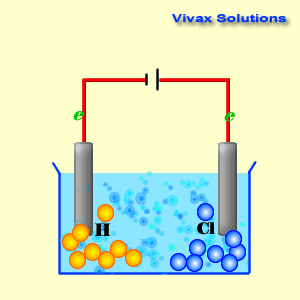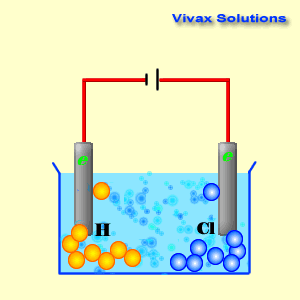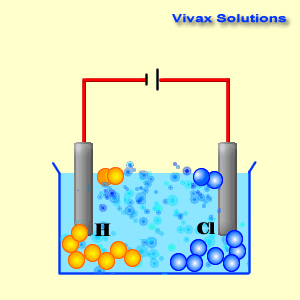
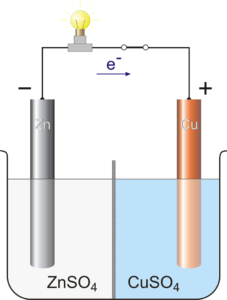
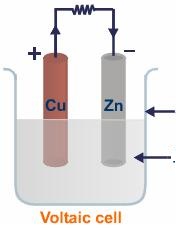
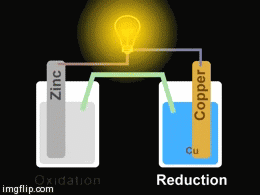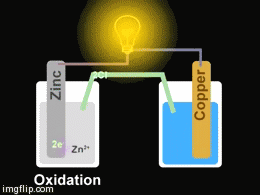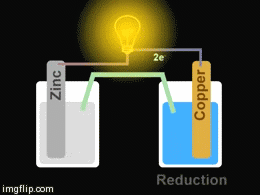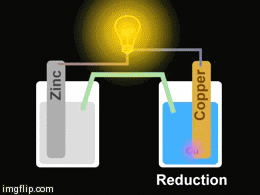
Voltaic cells. The first device to produce a steady, lasting electric current was the voltaic cell invented by Italian physicist Alessandro Volta. He created a voltaic pile in 1800 by stacking alternating copper and zinc disks separated by disks of cardboard soaked in saltwater. See Fig. When the top and bottom disks were connected by a wire an electric current (i.e. a stream of electrons) would flow through the wire. Fig. shows a voltaic cell consisting of a zinc rod and copper rod sitting in a solution of HCl. This cell will develop a potential difference of about 1.5 volts. A current (a stream of electrons) will flow from the negative zinc terminal to the positive copper terminal if the terminals are connected by a wire.
Batteries generally require several chemical reactions in order to operate. At least one reaction occurs in or around the anode and one or more reactions occur in or around the cathode. In all cases, the reaction at the anode produces extra electrons in a process called oxidation, and the reaction at the cathode uses the extra electrons during a process known as reduction.
 Leclanche’s battery
Leclanche’s battery
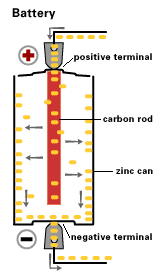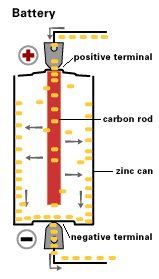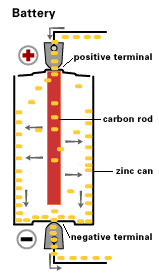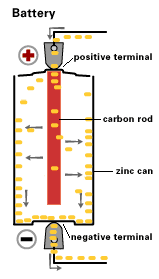
By 1866, Georges Leclanche, a French engineer, patented a new system, which was immediately successful. In the space of two years, twenty thousand of his cells were being used in the telegraph system. Leclanche’s original cell was assembled in a porous pot. The positive electrode consisted of crushed manganese dioxide with a little carbon mixed in. The negative pole was a zinc rod. The cathode was packed into the pot, and a carbon rod was inserted to act as a currency collector. The anode or zinc rod and the pot were then immersed in an ammonium chloride solution. The liquid acted as the electrolyte, readily seeping through the porous cup and making contact with the cathode material. Leclanche’s “wet” cell (as it was popularly referred to) became the forerunner to the world’s first widely used battery, the zinc carbon cell. Plante Battery- Leclanche’s invention, which was quite heavy and prone to breakage, was steadily improved over the years.
Secondary Batteries
 This type of battery must be charged after it is constructed Secondary cells, or accumulators may be re-charged many times over. The most common type of secondary cell is the “lead-acid” type used to start motor car engines. Taken to the extreme lead-acid batteries can produce a very high output for a short duration. These batteries are the least expensive but have a limited life and store less energy per pound than other types. Bharat Automotive Series It is important to note that nearly all of the batteries commonly used in deep cycle applications are Lead-Acid. This includes the standard flooded (wet) batteries, gelled, and AGM. They all use the same chemistry, although the actual construction of the plates etc can vary considerably. Ni-Cads, Nickel-Iron, and other types are found in some systems, but are not common due to their expense and/or poor efficiency.
This type of battery must be charged after it is constructed Secondary cells, or accumulators may be re-charged many times over. The most common type of secondary cell is the “lead-acid” type used to start motor car engines. Taken to the extreme lead-acid batteries can produce a very high output for a short duration. These batteries are the least expensive but have a limited life and store less energy per pound than other types. Bharat Automotive Series It is important to note that nearly all of the batteries commonly used in deep cycle applications are Lead-Acid. This includes the standard flooded (wet) batteries, gelled, and AGM. They all use the same chemistry, although the actual construction of the plates etc can vary considerably. Ni-Cads, Nickel-Iron, and other types are found in some systems, but are not common due to their expense and/or poor efficiency.
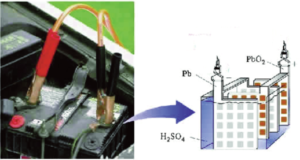
Types of Batteries
There are many ways to make a battery, some models are over 200 years old and others (such as those using carbon nanotubes) are being quickly advanced right at this moment!









Electrochemical cells. Electrochemical cells produce electricity through chemical reactions. We explained
Solar cells.A solar cell converts visible light into electrical current. It is based on the ability of certain semiconductor materials to generate small amounts of electric current when exposed to light. When light strikes the solar cell, electrons are knocked loose from the atoms of the semiconductor material. If the two sides of the solar cell are connected by a wire, an electric current will flow.
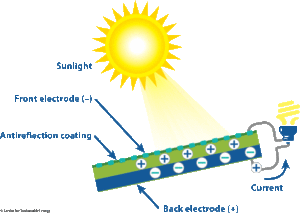
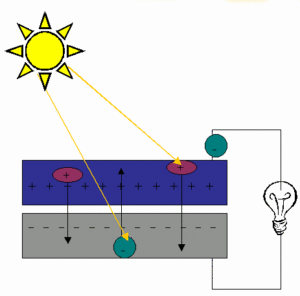 Thermocouples.If wires of two dissimilar metals (such as copper and iron) are joined at their ends, and these ends are maintained at different temperatures, a current will flow in the wires. A thermocouple is a device based on this fact. The voltage of the thermocouple depends on the two metals used and on the difference in temperature.
Thermocouples.If wires of two dissimilar metals (such as copper and iron) are joined at their ends, and these ends are maintained at different temperatures, a current will flow in the wires. A thermocouple is a device based on this fact. The voltage of the thermocouple depends on the two metals used and on the difference in temperature.
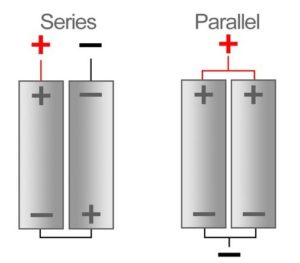
Series Parallel Batteries
Battery cells can be connected in series, in parallel and as well as a mixture of both the series and parallel.
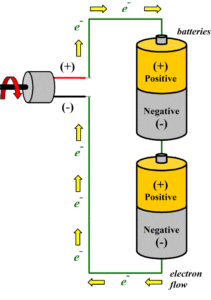
Series Batteries
When in a battery, positive terminal of one cell is connected with the negative terminal of succeeding cell, then the cells are said to be series connected or simply series battery. Here, overall emf of the battery is algebraic sum of all individual cells connected in series. But overall discharged electric current of the battery does not exceed the discharged electric current of individual cells.
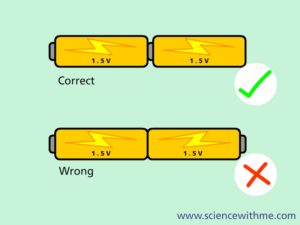

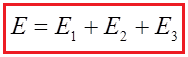
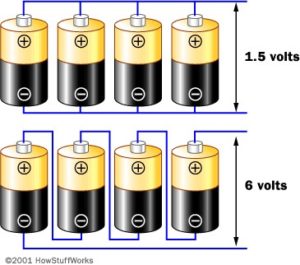
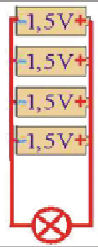
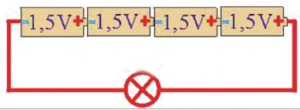 Example 1: The figure shows the serial connection of 4 battery voltages of 1.5 V
Example 1: The figure shows the serial connection of 4 battery voltages of 1.5 V
The total voltage of this battery is:
![]()
![]()
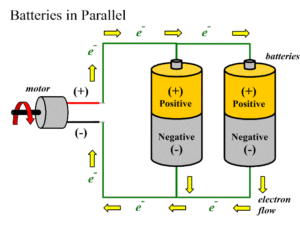 Parallel Batteries
Parallel Batteries
When positive terminals of all cells are connected together and similarly negative terminals of these cells are connected together in a battery , then the cells are said to be connected in parallel. These combinations are also referred as parallel battery.

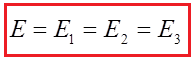
Example 2: The image shows a parallel connection 4 battery voltages of 1.5 V. The total voltage of this battery is:
![]()

History of Battery
Pre-electrical age batteries:
The history of the circuit starts with the history of the battery. It is worth mentioning that there may have been batteries prior to the modern electrical age. Since these are disconnected from the main timeline of electrical history we have listed them here. “There were some pretty smart metal workers in the ancient city of Baghdad, Persia [now Iraq]. They did a lot of fine work in steel, gold, and silver. You may wonder what this had to do with electric batteries. It seems that copper vases, some of whose ages go back 4000 years, were unearthed several years ago which had designs plated on them in gold or silver, even some were plated with antimony.”
The Bagdad Battery
The Bagdad Battery
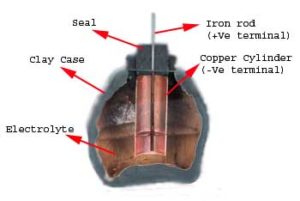 The first battery 248 BC: The Bagdad Battery was built in the Parthian or Sassanid period ~248 BC – 226 AD. The battery consisted of a carbon rod in the center of a clay vase. The rod was surrounded by an unknown electrolyte (likely to be orange/lemon juice), then copper, then asphaltum. Each battery had a weight of about 2 kilograms and produced 0.4-0.5 volts with open contacts. These batteries were very weak. The “Bagdad Battery” was found in 1936 and is believed to be authentic by many reputable sources.
The first battery 248 BC: The Bagdad Battery was built in the Parthian or Sassanid period ~248 BC – 226 AD. The battery consisted of a carbon rod in the center of a clay vase. The rod was surrounded by an unknown electrolyte (likely to be orange/lemon juice), then copper, then asphaltum. Each battery had a weight of about 2 kilograms and produced 0.4-0.5 volts with open contacts. These batteries were very weak. The “Bagdad Battery” was found in 1936 and is believed to be authentic by many reputable sources.
Ark of the Covenant
Ark of the Covenant
 The Egyptians: Some claim that the ancient Egyptians had batteries similar to the Bagdad Battery. The Ark of the Covenant: It has been theorized that the Ark of the Covenant (a gold lined box) may have used early batteries to energize the gold exterior. The box would then be able to give the illusion of magic powers by shocking those who touched it. This is only a theory, but would be an interesting use of electricity to create a sense of awe and fear.
The Egyptians: Some claim that the ancient Egyptians had batteries similar to the Bagdad Battery. The Ark of the Covenant: It has been theorized that the Ark of the Covenant (a gold lined box) may have used early batteries to energize the gold exterior. The box would then be able to give the illusion of magic powers by shocking those who touched it. This is only a theory, but would be an interesting use of electricity to create a sense of awe and fear.
New history
Luigi Galvani

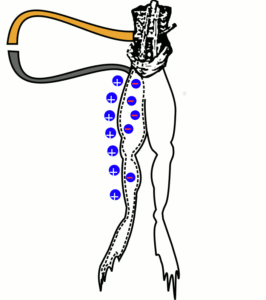
During the 1780’s, biologist Luigi Galvani performed experiments at the University of Bologna involving frogs. While cutting a frog’s leg, Galvani’s steel scalpel touched a brass hook that was holding the leg in place. The leg twitched. Further experiments confirmed this effect, and Galvani was convinced that he was seeing the effects of what he called animal electricity, the life force within the muscles of the frog.
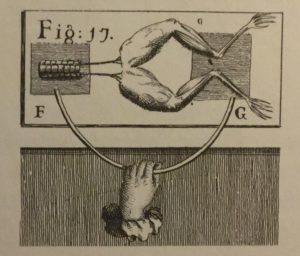


In the disputed experiment, Galvani had connected a frog’s leg to its spinal cord using two different pieces of metal (these metals were usually iron and bronze or iron and silver). Upon touching the metals to complete the circuit, the frog’s leg twitched. Galvani concluded that electricity was intrinsic to the frog and the metals simply acted as a conduit.
Alessandro Volta
 At the University of Pavia, Galvani’s colleague Alessandro Volta was able to reproduce the results, but was skeptical of Galvani’s explanation.
At the University of Pavia, Galvani’s colleague Alessandro Volta was able to reproduce the results, but was skeptical of Galvani’s explanation.
By experiment Volta found that it was the two dissimilar metals, not the frog’s leg that produced the electicity. The frog’s leg was just an indicator of presence of the electricity. In 1799 the Italian physicist Alessandro Volta created the first battery that emitted a steady, lasting current. He created it by alternating zinc and silver disks, one on top of the other, that were separated by saltwater soaked cardboard disks. This wet cell battery originally was named the “artificial electrical organ” for its association with the popular idea that electricity was generated by animal tissue, such as that from the electric torpedo fish. The invention was later named the “Voltaic Pile.”
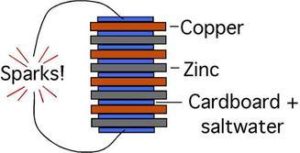
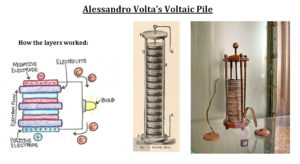 His colleagues further immortalized him by naming the units of electromagnetic force that emitted from the contraption “volts.” Volta’s invention was the first modern success in battery technology, but it still wasn’t able to sustain an electrical current long enough to power objects.
His colleagues further immortalized him by naming the units of electromagnetic force that emitted from the contraption “volts.” Volta’s invention was the first modern success in battery technology, but it still wasn’t able to sustain an electrical current long enough to power objects.
A compact version of this, using a stack of discs of copper, zinc & cardboard moistened with salt solution, was shown to Napolean in 1801. Upon presenting his invention, Napolean awarded Volta the medal of the Legion of Honor and made Volta a count.